Chromatography
MetAmino FAQ
What dilutions solvent shall I use for AA standards dilution?
The preferred dilution medium for AA standards is AASDM (Amino acids standard dilution medium), but pure deionized water is also suitable. The „Dilution and washing medium“ has a different function in the kit.
What is the type of membrane inside the spin filter?
- The membrane is made of NYLON material and its porosity is 0.22 µm. Its diameter is optimized for use with a given spin filter.
It is possible to expand the MetAmino® kit with other analytes.
- Yes, the MetAmino® kit can be further expanded. Contact us with detailed information.
May I use the kit for urine as well?
- Yes, the MetAmino® kit can be used with urine. The matrix should be without proteins. So, the sample has to be prepared accordingly (centrifugation, filtration).
Can we order the reagents separately?
- Yes, the entire set of reagents can be ordered under catalog number MAK-5857-L002.
What is the back pressure of the LC/MS column?
- The pressure is 380 bars during the beginning of the analysis, at the end it is 200 bars.
Are MRMs for amino acids given in the MetAmino® Kit were made after derivatization of the standards or before?
- The MRM transitions for AAs given in the kit manual are given as transitions of the AA derivatives and not of the native AA
Should the sample of feed be hydrolyzed before working with the MetAmino® Kit?
- Precipitation with Precipitation medium (PM) is not an essential step for successful derivatization of the sample. The kit was tested mainly for the analysis of biofluids, which often contain peptides. To avoid their precipitation during derivatization, the precipitation step was included in the sample preparation protocol.
- A total analysis of the free and peptide-bound amino acids is required. In this case, we would recommend hydrolyzing the sample and then drying the aliquot of hydrolyzed sample under a nitrogen stream or in a speedvac. We would then dissolve the dried residue in 25 µL of deionized water or 0.1M aqueous HCl (better solubility) and follow the procedure in the manual by adding 10 µL of the IS solution.
- As for the peptide hydrolysis, the additives (phenol, thiodiglycol) in the hydrolysis medium could also be derivatized (although they are probably not visible in the full scan). Therefore, we would simply recommend 6M HCl as the hydrolysis medium.
Can we order a MetAmino® GC/MS sample preparation kit for 400 samples?
- Not yet, we currently only have a MetAmino® GC/MS sample preparation kit for 100 samples. The kit can be ordered under catalog number MAK-5857-BA01.
There are 78 amino acids in table 1, some of which are not in the available mixes of standards - should we therefore rely on MRM transitions and look at the retention time or are these the standards that we should buy if we want to determine them?
- For quantification purposes the MetAmino kit contains - one bottle of SD1 standard solution included in the kit contains 33 amino acids: AAA, ABA, ALA, APA, ARG, ASP, BAIBA, CC, CIT, CTH, GABA, GLU, GLY, GPR, HIS, HLY, HYP, ILE, LEU, LYS, MET, 1MHIS, 3MHIS, ORN, PHE, PHP, PRO, SAR, SER, THR, TPR, TYR, VAL and bottle of SD2 with lyophilized mixture of 3 amino acids: ASN, GLN, TRP. However, the amino acid set can further be expanded to other compounds containing primary or secondary amino functional groups.
- The masses (m/z) reported in the manual are correct and represent M+H+ ions. The masses (m/z) and the retention times from the manual were obtained from the linear ion trap LTQ instrument however, the data from the Certificate of analysis were obtained from Q Exactive plus HRMS instrument. In both cases, the same column, flow rate, and mobile phase composition were used. The retention times (RT) are experimental values and the discrepancy especially for late eluting compounds would be caused by using different LC/MS instruments.
How can I predict how much to dilute a sample?
- It depends on the concentration of analyzed amino acids in the sample. The total amount of amino acids in the sample taken to the reaction should not exceed 1.2 μmoles. The amount of sample also depends on the LCMS instrument.
How much sample we need to take to hydrolisys with 6M HCl to use it with MetAmino® kit?
- You can take a higher amount of sample for AA hydrolysis (e.g. 50 micrograms in 300 microliters of 6M HCl) and finally take a few microliters (remove the acid in the speedvac or under the nitrogen stream) for the Metamino workflow.
In which point should the sample be diluted? After hydrolysis of the sample and before applying the kit? Or is it possible after the complete application of the kit (finished sample)?
- The reaction is designed to analyze aqueous samples, so if 25 μL of the sample gives an overloaded signal, I would recommend diluting the sample with deionized water. It is also possible to dilute the finished sample.
How much derivatizing reagent is used during derivatization process of the sample? How can I be sure that the reaction is 100%?
- The derivatization agent is sufficient to derivatize 1.2 μmol total amount of amino acids. The derivatization and the purification don´t always proceed at 100%. If you perform the derivatization of samples and calibration points in the same way, you should obtain the correct values. (Even if the effectivity of the derivatization and the micro spin purification is lower than 100%).
HPLC Phases
ASTRA - CHROMSERVIS
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| C18-HE | 2, 3, 5, 10 | 100 | 330 | 17 | 2-9 |
| C18-AQ | 2. 3, 5 | 100 | 330 | 13 | 2-9 |
| C18-BDS | 3, 5 | 140 | 170 | 11 | 2-8 |
| C8-HE | 5 | 100 | 330 | 11 | 2-9 |
| C8-BDS | 3, 5 | 140 | 170 | 6 | 2-8 |
| Phenyl-Hexyl-HE | 3, 5 | 100 | 330 | 11 | 2-7.5 |
| DM | 3, 5 | 100 | 205 | 12 | 2-9 |
| Diol | 3, 5 | 100 | 330 | 5 | 2-7.5 |
| Si | 3, 5 | 100 | 330 | - | 2-8 |
ARION - CHROMSERVIS
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| Plus C18 | 1.7, 2.2, 3, 5, 10, 15 | 100 | 420 | 18 | 1.5-10 |
| Polar C18 | 2.2, 3, 5, 10, 15 | 120 | 325 | 16 | 1.5-7.0 |
| C8 | 3, 5 | 120 | 325 | 11 | 2.0-7.0 |
| Phenyl-butyl | 2.2, 3, 5 | 100 | 300 | 12 | 1.5-7.5 |
| NH2 | 2.2, 3, 5 | 120 | 325 | 5 | 2.0-6.5 |
| CN | 3, 5, 10 | 120 | 325 | 8 | 2.0-7.0 |
| HILIC Plus | 2.2, 3, 5 | 120 | 420 | - | 1.5-7.0 |
| Si | 2.2, 3, 5, 10 | 100 | 420 | - | 1.5-7.0 |
| SAX | 5 | 120 | 325 | - | 1.0-7.5 |
| SCX | 5 | 120 | 325 | - | 1.0-7.5 |
More information is available in Column care guide aswell.
CHROMSHELL - CHROMSERVIS
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| CHROMSHELL® C18 Plus | 2.6 | 85 | 130 | 9 | 1.5-7.5 |
| CHROMSHELL® C18-XB | 2.6 | 85 | 130 | 8 | 1.5-8.0 |
| CHROMSHELL® C18 Polar | 2.6 | 85 | 130 | 6.5 | 1.5-7.0 |
KINETEX - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Effective Surface Area (m2/g) | Carbon Load (%) | pH Range |
|---|---|---|---|---|---|
| Kinetex XB-C18 | 5, 2.6 | 100 | 200 | 10 | 1.5-8.5* |
| Kinetex C18 | 5, 2.6 | 100 | 200 | 12 | 1.5-8.5* |
| Kinetex C8 | 2.6 | 100 | 200 | 8 | 1.5-8.5* |
| Kinetex PFP | 5, 2.6 | 100 | 200 | 9 | 1.5-8.5* |
| Kinetex HILIC | 2.6 | 100 | 200 | 0 | 2.0-7.5 |
| Kinetex Phenyl-Hexyl | 5, 2.6 | 100 | 200 | 11 | 1.5-8.5* |
* Columns are pH stable from 1.5 to 10 under isocratic conditions. Columns are pH stable from 1.5 to 8.5 under gradient conditions.
Kinetex 2.6µm columns with ID 2.1mm are pressure stable up to 1000 bar, otherwise up to 600 bar.
Kinetec chore-shell colums can be replace by new ChromShell colums. Just try it.
LUNA - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Luna Phenyl-Hexyl | 3,5,10,15 | 100 | 400 | 17.5 | 1.5-10.0 | L11 |
| Luna Silica (2) | 3,5,10,15 | 100 | 400 | - | - | L3 |
| Luna C5 | 5,10 | 100 | 440 | 12.5 | 1.5-10.0 | - |
| Luna C8 | 5,10 | 100 | 440 | 14.75 | 1.5-10.0 | L7 |
| Luna C8 (2) | 3,5,10,15 | 100 | 400 | 13.5 | 1.5-10.0 | L7 |
| Luna C18 | 5,10 | 100 | 440 | 19 | 1.5-10.0 | L1 |
| Luna C18 (2) | 2.5,3,5,10,15 | 100 | 400 | 17.5 | 1.5-10.0 | L1 |
| Luna CN | 3,5,10 | 100 | 400 | 7.0 | 1.5-10.0 | L10 |
| Luna NH2 | 3,5,10 | 100 | 400 | 9.5 | 1.5-11.0 | L8 |
| Luna SCX | 5,10 | 100 | 400 | 0.55% Sulfur Load | 2.0-7.0 | L9 |
| Luna HILIC | 3,5 | 200 | 200 | - | 1.5-8.0 | - |
| Luna PFP(2) | 3 5 | 100 | 400 | 5.7 | 1.5-8.0 | L43 |
GEMINI - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Gemini C18 | 3,5,10 | 110 | 375 | 14 | 1.0-12.0 | L1 |
| Gemini C6-Phenyl | 3,5 | 110 | 375 | 12 | 1.0-12.0 | L11 |
| Gemini NX | 3,5,10 | 110 | 375 | 14 | 1.0-12.0 | L1 |
SYNERGI - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Synergi Max-RP | 2.5 | 100 | 400 | 17 | 1.5-10.0 | - |
| Synergi Hydro-RP | 2.5 | 100 | 400 | 19 | 1.5-7.5 | L1 |
| Synergi Polar-RP | 2.5 | 100 | 440 | 11 | 1.5-7.0 | L11 |
| Synergi Fusion-RP | 2.5 | 100 | 440 | 12 | 1.5-10.0 | L1 |
| Synergi Max-RP | 4,10 | 80 | 475 | 17 | 1.5-10.0 | - |
| Synergi Hydro-RP | 4,10 | 80 | 475 | 19 | 1.5-7.5 | L1 |
| Synergi Polar-RP | 4,10 | 80 | 475 | 11 | 1.5-7.0 | L11 |
| Synergi Fusion-RP | 4,10 | 80 | 475 | 12 | 1.5-10.0 | L1 |
ONYX - PHENOMENEX
| Packing Material | Macropore Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Onyx Silica | 2 | 130 | 300 | 0 | 2.0-7.5 | - |
| Onyx C8 | 2 | 130 | 300 | 11 | 2.0-7.5 | - |
| Onyx C18 | 2 | 130 | 300 | 18 | 2.0-7.5 | - |
JUPITER - PHENOMENEX
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| Jupiter C4 | 5,10,15 | 300 | 170 | 5.0 | 1.5-10.0 | L26 |
| Jupiter C5 | 5,10,15 | 300 | 170 | 5.5 | 1.5-10.0 | - |
| Jupiter C18 | 5,10,15 | 300 | 170 | 13.3 | 1.5-10.0 | L1 |
| Jupiter Proteo C12 | 4,10 | 90 | 475 | 15.0 | 1.5-10.0 | - |
Nano / Capillary LC Column ProteCol - SGE
| Packing Material | Particle Size (µm) | Pore Size (Å) | Surface Area (m2/g) | Carbon Load (%) | pH Range | USP Packing |
|---|---|---|---|---|---|---|
| ProteCol C18 | 3 | 120/300 | 350 | 17 | 2.0-7.5 | L1 |
| ProteCol C8 | 3 | 120/300 | 350 | 10 | 2.0-7.5 | L7 |
| ProteCol C4 | 3 | 120/300 | 350 | 2.0-7.5 | L26 | |
| ProteCol SCX | 3 | 120/300 | 350 | 2.0-7.5 | L9 |
Capillary GC column installation
 Brief procedure for column installation
Brief procedure for column installation
- Cool all heated zones of GC
- Check gas purifiers and if they are spent, replace them.
- Clean injector and detector
- Replace injector/detector liners with new ones.
- Replace critical injector and detector seals.
- Replace septum in the injector.
- Set make-up and detector gas flow rates.
- Carefully inspect the column for damage or breakage.
- Install a nut and ferrule on each end of the column.
- Cut 10 centimeters from each end of the column. Use a sapphire scribe or ceramic scoring wafer to cut fused silica capillary columns. Use the serrated edge of a ceramic scoring wafer or the edge of a sharp file to cut metal capillary columns. See our catalogue for capillary cuting tools.
- Mount the capillary column in the oven using a bracket that protects the column from becoming scratched or abraded.
- Insert column the appropriate distance into the inlet as indicated in the instrument manual.
- Install the column so that the capillary does not touch the oven walls.
- Set the approximate column flow rate by adjusting the head pressure to the value listed on the test chromatogram included with the column.
- Set split vent, septa purge, and any other applicable inlet gases according to the instrument specifications.
- Confirm the flow by immersing the column outlet in a vial of solvent (acetone or isopropyl alcohol).
- Insert column the appropriate distance into the detector as indicated in the instrument manual.
- Check for inlet and outlet leaks using a thermal conductivity leak detector. Do not use soaps or liquid-based leak detectors or the column may be damaged.
- Set injector and detector temperatures. Turn the detector on when the temperatures have equilibrated. Caution - do not exceed the phase's maximum operating temperature!
- To set the proper dead time (linear velocity), inject methane or a non-retained substance compatible with the detector being used.
- Verify system integrity by checking the dead volume peak. It should not tail.
- Condition the column at its maximum operating temperature to stabilize the baseline. (See the test chromatogram included with the column for the maximum temperature.)
- Set oven to appropriate temperature and inject methane or an appropriate unretained substance, again to set the proper linear velocity.
- Inject a duplicate of the original test mixture or your specific test mixture to confirm proper installation, system, and column performance.
- Calibrate the instrument and inject samples.
Note: If the column is new, you have to run conditioning procedure prior setting the proper dead time.
GC Column conditioning
 Conditioning at elevated temperatures without flow will permanently damage or destroy the performance of the capillary column. Conditioning with an oxygen leak present causes the column bleed and destroys its utility at high operating temperatures. Therefore prior the conditioning a column:
Conditioning at elevated temperatures without flow will permanently damage or destroy the performance of the capillary column. Conditioning with an oxygen leak present causes the column bleed and destroys its utility at high operating temperatures. Therefore prior the conditioning a column:
- Check proper carrier gas flow settings
- Make a leak test to verify that there is not oxygen present in the system
GC column conditioning:
- Install the column to the injector. Do not connect the column to the detector. Leave the column end in the GC oven.
- Set the GC oven to 40°C
- Hold this temperature 15 minutes
- Set the temperature gradient to 10°C/min
- Set the maximum operation temperature 20°C above your final temperature of the GC temperature program (the maximum temerature must be 25°C below the column maximum operating temperature)*
- Leave the column at high temperature ovenight until the baseline stabilizes. If the column is pre-conditioned, leave tha maximum temperature for 2 hours and untill the baseline stabilizes.
*Note: Overnight conditioning is not necessary with pre-conditioned columns. Read carefully the conditioning instructions supplied by the column manufacturer. They have priority to the general conditioning recommendation above .
EasyFlash columns
Chromservis has launched new EasyFlash columns for high efficient flash chromatography. In this article, you will learn about the Flash chromatography benefits.
HPLC connections (TN #539)
This technical note shows possible types of connection between column and LC system. Bad connection may influence peak separation and should be avoided. The right connection is presented below.

- TN_539_HPLC connections
1791 kB
How the Triple Quadrupole works?
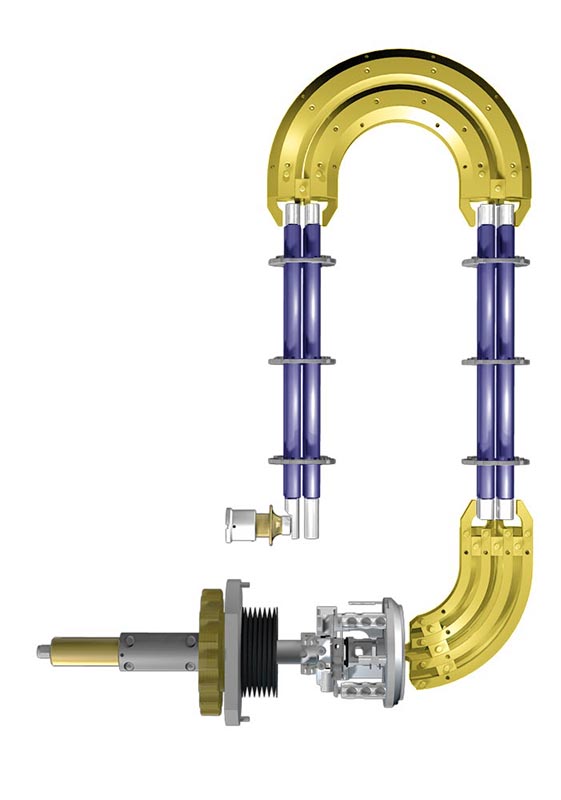 The principle of Triple Quadrupole (TQ) is explained on EVOQ™ system by Bruker. The key points of the system are:
The principle of Triple Quadrupole (TQ) is explained on EVOQ™ system by Bruker. The key points of the system are:
- Axial Ion Source
- Active Focusing Q1
- Lens-Free Ion Path
- 180° Collision Cell
- Elliptical Design
- Off Axis Detector
puriFlash
puriFlash® Flash Cartridges
Interchim developed new technique for flash chromatography - Ultra Performance Flash Purification (UPFP) using special flash cartridges. The flash cartridges are in the form of regular or irregular silica. UPFP enables to run purifications with high purity of the yield and less solvent use.
Preparative LC
 Task for preparative HPLC systems differs to analytical one. While analytical HPLC task is qualitative and quantitative determination of defined compounds in samples, preparative HPLC task is separation, purification and isolation of value products from mixtures.
Task for preparative HPLC systems differs to analytical one. While analytical HPLC task is qualitative and quantitative determination of defined compounds in samples, preparative HPLC task is separation, purification and isolation of value products from mixtures.
Preparative chromatography can be devidet into three main areas:
- Semi-preparative separations
- Batch preparative chromatography (pilot or industrial scale)
- True Counter-current chromatography
- Simulated moving bed (SMB)
- Continuous chromatography
Scale definition
| Parameter | Analytical | Semi-preparative | Preparative |
|---|---|---|---|
| Column sizes (mm) | 120 - 250 x 2 - 4.6 | 120 - 250 x 8 - 16 | 120 - 250 x 20 - 62 |
| Particle size (µm) | up to 5 | 5 - 10 | higher than 10 |
| Stationary phase (g) | up to 5 | 5 - 30 | 50 - 450 |
| Tubings | 1/16" | 1/16" | 1/8" |
| Flow rates (ml/min) | 0.1 - 2 | 5 - 50 | 100 - 1000 |
| Sample size (mg) | 0.01 - 2 | 0.1 - 50 | 1 - 700 |
| Flow cell (mm) | 10 | 3 | 0.5 - 2 |
Preparative chromatography can be conbined with Flash chromatography in one system - purification device. The PuriFlash (Advion-Interchim) offers different modes of operation:
- Preparative line + Flash line
- Two Flash lines
- Two Preparative lines.
Introduction
Amino acids are key building blocks of life and play pivotal role in various metabolic pathways. They mostly act as intermediates often not directly involving proteins. Due to their chemical complexity and dynamic range, their reliable quantitative and qualitative analysis in biological fluids and tissues is crucial for nutritional information, compound identification and diagnostics.
For that purpose, a simple, elegant and thus far the most expeditious method for an invaluable amino acid analysis has been developed. LC/GC-MS based Metamino® kit offers comprehensive solution up to 75 metabolites including basic proteinogenic amino acids, biogenic amines and coenzymes with the possibility to a further analyte extension.
- MetAmino_brochure 2023_EN
3480 kB





 0
0
 0
0