
Gas Detection
Dryers table - quick guide
Dryer table - quick guide for selection of dryer -basic parameters.
| Parameter / Model | MD | PD | MDH (heated) | ME | DM (portable) |
|---|---|---|---|---|---|
| Type | Single tube | Multitubes (50, 100, 200) | Single tube | Single tube | Single tube + dessicant |
| Nafion tube O.D. | 0,053", 0,072", 0,108" | - | 0,108" | 0,053", 0,063", 0,072", 0,108" | - |
| Nafion tube I.D. | 0,042", 0,060", 0,086" | - | 0,086" | - | 0,052," 0,086" |
| Available Lengths (inch) | 12, 24, 48, 72, 96, 144 | 12, 24, 48, 72 | 96 | 6, 12, 18 24, 36, 48 | 24 |
| Housing Materials | Stainless Steel or Fluorocarbon or Polypropylene | Stainless Steel or Fluorocarbon or Polypropylene | Flourocarbon | Polypropylene | - |
| Maximum Flow Rate | 200 ml/min, 2, 4 l/min depending of type | 4, 8 nebo 15 l/min depending of type | 1 l/min | 1,2 l/min | 0,5; 1 l/min depending of type |
Parameters and recomandation for dryers MD - dryer with one tube.
| Parameter | MD-050 | MD-070 | MD-110 |
|---|---|---|---|
| Nafion tube O.D. | 0,053" | 0,072" | 0,108" |
| Nafion tube I.D. | 0,042" | 0,060" | 0,086" |
| Available Lengths (inch) | 12, 24, 48, 72 | 12, 24, 48, 72, 96, 144 | |
| Housing Materials | SS, Flourocarbone, PP | ||
| Maximum Flow Rate | 200 ml/min | 2 l/min* | 4 l/min* |
* MD-070 and MD-110 offer approximately the same drying performance. Specify MD-110 when pressure drop is a concern, MD-070 to minimize dead volume. For higher fl ow rates, please see our PD-Series dryers.
Parameters and recomandation for dryers PD - dryer with more tube.
| Parameter | PD-050T | PD-100T | PD-200T |
|---|---|---|---|
| Number of nafion tubes | 50 | 100 | 200 |
| Available Lengths (inch) | 12, 24, 48, 72 | ||
| Housing Materials | SS, Flourocarbon, PP | ||
| Recommended Flow Rates* | 4 l/min | 8 l/min | 15 l/min |
* Flow rates based upon unheated, 24" dryer achieving a -10°C dew point.
Nafion dryers
 Perma Pure LLC manufactures components and devices primarily designed to dry and humidify gas streams. The core technology is Nafion®, a Dupont co-polymer that is highly selective in the removal of water. Perma Pure manufactures Nafion into tubing of various diameters to optimize the transfer of water through the Nafion. The water moves through the membrane wall and evaporates into the surrounding air or gas in a process called perevaporation. This process is driven by the humidity gradient between the inside and the outside of the tubing. In addition to drying and humidifying, our products can be used as ion exchange membranes and specialty separation membranes that take advantage of the unique properties of Nafion.
Perma Pure LLC manufactures components and devices primarily designed to dry and humidify gas streams. The core technology is Nafion®, a Dupont co-polymer that is highly selective in the removal of water. Perma Pure manufactures Nafion into tubing of various diameters to optimize the transfer of water through the Nafion. The water moves through the membrane wall and evaporates into the surrounding air or gas in a process called perevaporation. This process is driven by the humidity gradient between the inside and the outside of the tubing. In addition to drying and humidifying, our products can be used as ion exchange membranes and specialty separation membranes that take advantage of the unique properties of Nafion.
What is Nafion?
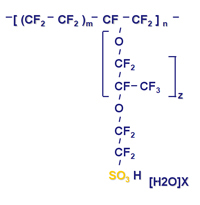 Nafion is a copolymer of tetrafluoroethylene (Teflon®) and perfluoro-3,6-dioxa-4-methyl-7-octene-sulfonic acid. Like Teflon, Nafion is highly resistant to chemical attack, but the presence of its exposed sulfonic acid groups confers unusual properties. Sulfonic acid has a very high water-of-hydration, absorbing 13 molecules of water for every sulfonic acid group in the polymer; consequently, Nafion absorbs 22% by weight of water.
Nafion is a copolymer of tetrafluoroethylene (Teflon®) and perfluoro-3,6-dioxa-4-methyl-7-octene-sulfonic acid. Like Teflon, Nafion is highly resistant to chemical attack, but the presence of its exposed sulfonic acid groups confers unusual properties. Sulfonic acid has a very high water-of-hydration, absorbing 13 molecules of water for every sulfonic acid group in the polymer; consequently, Nafion absorbs 22% by weight of water.
Unlike micro-porous membrane permeation, which transfers water through a relatively slow diffusion process, Nafion removes water by absorption as water-of-hydration. This absorption occurs as a First Order Kinetic reaction, so equilibrium is reached very quickly (typically with in milliseconds). Because this is a specific chemical reaction with water, gases being dried or processed are usually entirely unaffected.
Pellistor
Principle
 Pellistor is based on two thin platinum wires in aluminium beds connected to Wheatson bridge. One bed is covered with a special catalyst initiating oxidation of flammable gase (vapours), the other bed is modified to inhibit oxidation. The catalytic bed increases the temperature during the oxidation process in the presence of flammable gases (vapours). Oxidation process increases temperature of the bed with catalyst, which causes increase of resistance. The result of this status a bridge imbalance.
Pellistor is based on two thin platinum wires in aluminium beds connected to Wheatson bridge. One bed is covered with a special catalyst initiating oxidation of flammable gase (vapours), the other bed is modified to inhibit oxidation. The catalytic bed increases the temperature during the oxidation process in the presence of flammable gases (vapours). Oxidation process increases temperature of the bed with catalyst, which causes increase of resistance. The result of this status a bridge imbalance.
Benefits
- Linearn output up to 100% LEL
- Cheap and stable sensor
- Quick response time (< 10 s)
- Operation temperature: -40 to +60°C
Weaknesses
- Liable to poisoning and sensitivity decrease
- Required atmosphere with oxygen above 10% vol.
- Poisoned sensor gives output like sensor at zero concentration - requires regular span gas application
- Higher power consumption
Electrochemical
 Principle
Principle
Electrochemical sensor consists of 2, 3 or 4 electrodes, which are located in a gel electrolyte. A system of electrodes and the electrolyte is separated from the atmosphere by a diffusion barrier. Gas molecules diffuse through this barrier and react with the electrolyte. There are oxidation and reduction reactions at the electrodes and they cause a change of cell potential. The higher the gas concentration is, the higher the potential will be.
Benefits
- Reliable and non-expensive sensors for „common“ gases
Weaknesses
- Long response time (up to few minutes for some gases)
- Higher price for special gases
- Potential failure when exposed with high concentrations
- Cross interferences (ozone sensor is sensitive to air flow, temperature and humidity changes)
Photoionization detector
Principle
 A Photoionization detector (PID) uses an ultraviolet (UV) light source to ionize chemicals to positive and negative ions that can be easily counted with a detector. Ionization occurs when a molecule absorbs the light energy. The gas becomes electrically charged. These charged particles produce a current that is then amplified and displayed on the meter as "ppm" or even "ppb".
A Photoionization detector (PID) uses an ultraviolet (UV) light source to ionize chemicals to positive and negative ions that can be easily counted with a detector. Ionization occurs when a molecule absorbs the light energy. The gas becomes electrically charged. These charged particles produce a current that is then amplified and displayed on the meter as "ppm" or even "ppb".
Benefits
- modern type (3D) is not affected by temperature and humidity changes
- one detector can detect broad range of gases
- high sensitivity (ppb's)
- quick response (< 3 s)
- high precision at very low levels
Weaknesses
- low selectivity for most compounds
Infrared
Principle
A Infrared detector (IR) uses an ability of gases with two ore more atoms to absorb infrared (IR) light, e.g. carbon dioxide, methane. Infrared detectors use wavelengths specific corresponding to vibration or rotation of a bond between two atoms in the gas molecule. The higher gas concentration the lower IR signal (approx. logarithmic function).
Benefits
- Does not require oxygen for the measurement
- Impossible to damage by catalytic poisons
- DIrty optics warning
- Working up to 80% optics obscuration
- Good selectivity
Weaknesses
- Higher costs
TCD
 Principle
Principle
TC gas detectors, sometimes called Katharometers operate by comparing the thermal conductivity of the sample with that of a reference gas (usually air). A heated thermistor or platinum filament is mounted so that it is exposed to the sample and another, acting as a reference is enclosed in a sealed compartment.
If the sample has a higher thermal conductivity than the reference, heat is lost from the exposed element and its temperature decreases, whilst if the thermal conductivity is lower than that of the reference the temperature of the exposed element increases. These temperature changes cause electrical resistance changes, which are measured by means of a bridge circuit.
Because of the amount of power required to heat these sensors, they have to be mounted in a flameproof enclosure, in the same way as pellistors are. The precise mechanism, which occurs, is quite complex because the thermal conductivities of gases vary with temperature and convection as well as conductivity plays a role. Whilst most gases exhibit a linear output signal, this is not always the case.
Benefits
- Suitable for binary mixtures
- Fast response
Weaknesses
- Temperature dependent (long equilibration time)
- Inapplicable for more complex gas mixtures
- Gases with thermal conductivities of less than one are more difficult to measure, partly because water vapour may cause an interference problem
- Gases with thermal conductivities close to one cannot be measured (CO, O2, N2, NH3)
Cross references
 Electrochemical sensors are manufactured as specific sensors for one gas, for all that there exist some cross sensitivities to other gases. Generally these cross references are not a problem in gas detection in the area of safety, but they can be a complication in the process detection - they can cause false alarms. This page includes links to cross reference data of the electrochemical sensors.
Electrochemical sensors are manufactured as specific sensors for one gas, for all that there exist some cross sensitivities to other gases. Generally these cross references are not a problem in gas detection in the area of safety, but they can be a complication in the process detection - they can cause false alarms. This page includes links to cross reference data of the electrochemical sensors.
Pellistor correction factors for Crowcon portable detectors
Following technical note (DETC-TN-05) includes information about pellistor correction factors. This technical note is available here.
Gas Correction Factors
Correction factors of flammable gases for fixed detectors
Correction factors of Pellistor for Crowcon fixed detectors: click for open in pdf format





 0
0
 0
0