Váš košík je prázdný...


 0
Porovnat
0
Porovnat
 Uživatel
Uživatel
 0
Košík
0
Košík

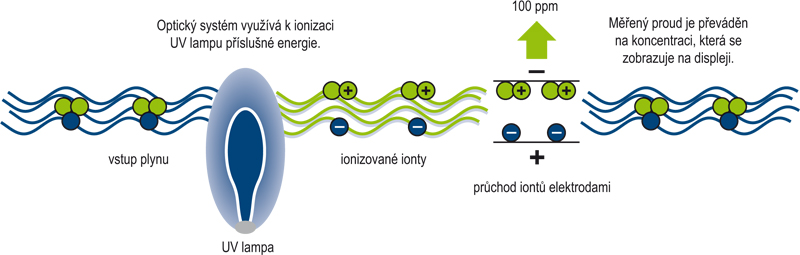
Fotoionizační detektor (Photo Ionization Detector) pracuje na principu měření elektrického náboje vzniklého při ionizaci měřeného plynu. U většiny plynů lze určit tzv. specifický ionizační potenciál (IP), který má jednotku eV. Měřený plyn je ionizován ultrafialovou zářivkou, což se projeví vznikem elektrického náboje. Ionizace plynu je však podmíněna skutečností, že ionizační potenciál plynu bude menší než hodnota potenciálu (eV) použité UV lampy (respektive energie vzniklých fotonů)! Vlastní senzor detekuje vzniklý náboj ionizovaného plynu a ten je převeden na elektrický proud. Proud je zesílem a převeden na koncentraci v jednotkách ppm nebo ppb.